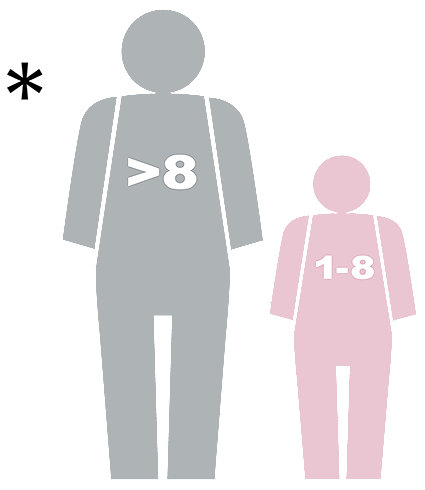Disclosure and safety information
Online access to Operating Instructions is provided at ifu.stryker.com for defibrillators and their accessories, and CPR assist devices at lucas-cpr.com for complete directions for use, indications, contraindications, warnings, precautions and potential adverse reactions. For further information, please contact Physio-Control at 1-800-442-1142.
Brief summaries
Brief summary of indications and important safety information
INDICATIONS FOR USE
DEFIBRILLATION The LIFEPAK 1000 is to be used in AED mode only on patients who are in cardiopulmonary arrest. The patient must be unresponsive, not breathing normally, and showing no signs of circulation. LIFEPAK 1000 may be used with standard defibrillation pads only on adults and children who are 8 years old or more or who weigh more than 25 kg (55 lbs). LIFEPAK 1000 may be used on children who are less than 8 years old or weigh less than 25 kg (55 lbs) with Infant/Child Reduced Energy Defibrillation Electrodes. ECG MONITORING is for use on conscious and unconscious patients of all ages for the purpose of ECG rhythm recognition and heart rate monitoring. CONTRAINDICATIONS: None.
OPERATOR CONSIDERATIONS: LIFEPAK 1000 requires operator interaction to defibrillate patient. It is intended for use by personnel who are authorized by a physician or medical director and have, at a minimum, the following skills and training: CPR training, defibrillator training equivalent to that recommended by American Heart Association, and training in use of defibrillator. LIFEPAK 1000 is intended for use in hospital and out-of-hospital environments. Manual mode is intended for use by personnel trained in ECG recognition who want to use defibrillator to deliver a shock independent of AED mode. Operator has control over charging and delivery of shocks. ECG mode provides a nondiagnostic ECG display and is intended for use by personnel trained in ECG recognition to allow for rhythm and heart rate monitoring using standard ECG electrodes. When in ECG mode, the defibrillator’s shock capability is disabled; however, LIFEPAK 1000 continues to analyze patient’s ECG for potentially shockable rhythm.
GENERAL/DEFIBRILLATION WARNINGS. Shock Hazards: LIFEPAK 1000 delivers up to 360 joules of electrical energy. Unless properly used, this electrical energy may cause serious injury or death. Do not attempt to operate unless thoroughly familiar with operating instructions and function of all controls, indicators, connections, and accessories. Clear everyone away from contact with patient, bed, and other conductive material before discharging defibrillator. When discharging defibrillator, do not touch electrodes. Do not immerse defibrillator in water or other fluids. Avoid spilling fluids on device or accessories. Do not disassemble defibrillator or its batteries. Contact authorized service personnel for repair. Possible skin burns and ineffective energy delivery: Dried out or damaged electrodes may cause electrical arcing and patient skin burns during defibrillation. Do not use electrodes that have been removed from foil package for >24 hours or expired electrodes. Check that electrode adhesive is intact and undamaged. Possible misinterpretation of ECG data: Do not analyze in a moving vehicle or move the AED during analysis. Motion artifact may affect ECG signal resulting in inappropriate shock or no shock advised message. Motion detection may delay analysis. Stop vehicle. Do not touch the patient or the AED during analysis.
Excessive Energy Delivery (AED mode): Do not use Pediatric QUIK-COMBO electrodes; these electrodes do not attenuate the energy delivery by LIFEPAK 1000. Implanted electrical devices: Defibrillation may interfere with implanted devices and cause them to malfunction. Place therapy electrodes away from implanted devices if possible. Possible defibrillator shutdown: Always have access to spare, fully-charged, properly maintained battery to avoid possible device shutdown without warning. Replace battery when LIFEPAK 1000 displays warning of REPLACE BATTERY. Possible device failure: Do not modify LIFEPAK 1000 or its batteries. Possible explosion, fire, noxious gas or burns: Do not use device in presence of flammable gases or anesthetics. Use care when operating close to oxygen sources. Turn off gas source or move source away from patient during defibrillation. Possible electrical interference or improper device performance: Use only parts and accessories specified by Physio-Control or Stryker. Using other manufacturers’ accessories may affect performance of device or equipment in close proximity and may invalidate safety agency certification. Defibrillator may cause electromagnetic interference (EMI) especially during charge and energy transfers which may affect performance of equipment operating in close proximity. Equipment operating in close proximity may emit strong EMI or radio frequency interference (RFI) which could affect performance of device. Recommended distances of equipment provided in Operating Instructions. Safety risk and possible equipment damage. Keep AED away from magnetic resonance imaging (MRI) equipment as it is unsafe. ECG MONITORING (ECG mode) WARNINGS. Possible delay in therapy: Do not attempt to connect 3-wire ECG cable to QUIK-COMBO therapy cable or any other AED. ECG cable is functional only with LIFEPAK 1000. Possible misinterpretation of ECG data: Frequency response of screen intended only for basic ECG rhythm identification; it does not provide resolution required for pacemaker pulse visibility, accurate measurements, such as QRS duration, and ST segment interpretation. For such purposes, use ECG monitors with appropriate frequency response.
GENERAL CAUTION: Possible equipment damage: Before using LIFEPAK 1000 disconnect all equipment that is not defibrillator-protected from patient.
U.S. Federal law restricts this device to sale by or on the order of a physician.
Please consult Operating Instructions at ifu.stryker.com or call 800.442.1142 for complete list of indications, contraindications, warnings, cautions, potential adverse events, safety and effectiveness data, instructions for use and other important information.
GDR 3338693_B
Brief summary of indications and important safety information
LIFEPAK 20e defibrillator/monitor is an acute cardiac care response system intended for use by authorized healthcare providers in hospital and clinic settings. It is to be used on one patient at a time. LIFEPAK 20e is intended for use by personnel who have been trained in its operation. AED MODE. INDICATIONS FOR USE: To be used only on patients in cardiopulmonary arrest. Patient must be unconscious, pulseless, and not breathing normally before using defibrillator to analyze patient’s ECG rhythm. In AED mode, LIFEPAK 20e is not intended for use on pediatric patients less than 8 years old.
CONTRAINDICATIONS: None known. OPERATOR CONSIDERATIONS: In AED mode, LIFEPAK 20e is intended for use by personnel authorized by physician/medical director and have, at a minimum, the following: CPR training, AED training equivalent to that recommended by AHA, and training in use of LIFEPAK 20e in AED mode. DEFIBRILLATION THERAPY. INDICATIONS FOR USE: Defibrillation is a recognized means of terminating certain potentially fatal arrhythmias, such as VF and symptomatic VT. Delivery of this energy in synchronized mode is a method for treating AF, atrial flutter, paroxysmal supraventricular tachycardia, and, in relatively stable patients, VT. CONTRAINDICATIONS: Treatment of PEA such as idioventricular or ventricular escape rhythms, and in treatment of asystole. OPERATOR CONSIDERATIONS: LIFEPAK 20e delivers energy through disposable electrodes, standard paddles applied to a patient's chest, or internal paddles applied directly to the patient's heart. Defibrillation is only one aspect of medical care required to resuscitate patient with shockable ECG rhythm. Other supportive measures may include CPR, administration of supplemental oxygen and drug therapy. NONINVASIVE PACING. INDICATIONS FOR USE: For symptomatic bradycardia in patients with pulse. CONTRAINDICATIONS: Treatment of VF and asystole. SPO2 MONITORING. Indications for Use: Pulse oximeter is for use in patient at risk of developing hypoxemia. Contraindications: None known. EtCO2 MONITORING. INDICATIONS FOR USE: To detect the level of expired CO2, used for monitoring breathing efficacy and treatment effectiveness in acute cardiopulmonary care, for example, to determine if adequate compressions are being performed during CPR or rapidly detect whether endotracheal tube has been placed successfully. CONTRAINDICATIONS: None known. LIFEPAK 20e with or without CodeManagement Module – ECG MONITORING: ECG obtained by placing either electrodes or paddles on patient; allows for heart’s electrical activity to be monitored and recorded.
Operating Instructions provide important information to help you operate LIFEPAK 20e and CodeManagement Module. Become familiar with all terms, warnings, and symbols. GENERAL/MANUAL DEFIBRILLATION/PADDLE WARNINGS and CAUTIONS: Shock or fire hazards. Possible explosion. Possible patient skin burns. Possible device or paddle damage. Possible device failure, inability to deliver therapy, ineffective energy delivery, shutdown, or improper device performance. Possible electrical interference with device performance, implanted electrical device or other equipment. Safety risk. Failure to detect change in ECG rhythm. Possible failure to detect out of range condition. AED WARNINGS: Possible misinterpretation of data. Pediatric patient safety risk. ECG MONITORING WARNING: Possible misinterpretation of ECG data. PEDIATRIC ECG MONITORING AND THERAPY PROCEDURES: Possible patient skin burns. SYNCHRONIZED CARDIOVERSION WARNING: Possible lethal arrhythmia. Possible monitor incompatibility. REMOTE SYNCHRONIZATION: Possible lethal arrhythmia. Possible monitor incompatibility. CPR METRONOME WARNING: CPR delivered when not needed. NONINVASIVE PACING WARNINGS: Possible inducement of VF. Possible inability to pace. Possible interruption of therapy. Possible patient skin burns. SPO2 WARNINGS AND CAUTION: Shock or burn hazard. Inaccurate pulse oximeter readings. Skin injury. Possible strangulation. Possible equipment damage. EtCO2 MONITORING WARNINGS AND CAUTION: Fire hazard. Possible inaccurate patient assessment or inaccurate CO2 readings. Possible strangulation. Infection hazard. Possible equipment damage. CODEMANAGEMENT MODULE BATTERY WARNING: Possible CO2 monitoring shutdown. REPLACING/REMOVING ELECTRODES WARNING: Possible cable damage and ineffective energy delivery or loss of monitoring.
U.S. Federal law restricts this device to sale by or on the order of a physician.
Please consult Operating Instructions at ifu.stryker.com or call 800.442.1142 for complete list of indications, contraindications, warnings, cautions, potential adverse events, safety and effectiveness data, instructions for use and other important information.
GDR 3338728_A
Brief summary of indications and important safety information
LIFEPAK 15 is a complete acute cardiac care response system designed for basic life support (BLS) and advanced life support (ALS) patient management protocols. INTENDED USE: LIFEPAK 15 intended for use by trained medical personnel out-of-doors, in indoor emergency care settings, and is designed to be used for ground transportation. Monitoring and therapy functions may only be used on one patient at a time. Manual mode monitoring and therapy functions are intended for use on adult and pediatric patients. Automated external defibrillation (AED) mode intended for use on patients ≥8 years of age.INDICATIONS FOR USE – MANUAL DEFIBRILLATION: Indicated for termination of certain potentially fatal arrhythmias, such as ventricular fibrillation and symptomatic ventricular tachycardia. Delivery of energy in synchronized mode is a method for treating atrial fibrillation, atrial flutter, paroxysmal supraventricular tachycardia and, in relatively stable patients, ventricular tachycardia. CONTRAINDICATIONS - MANUAL DEFIBRILLATION: Contraindicated in treatment of PEA and asystole. AED MODE: To be used only on patients in cardiopulmonary arrest. Patient must be unconscious, pulseless, and not breathing normally before using defibrillator to analyze patient’s ECG rhythm. In AED mode, the LIFEPAK 15 is intended for use on pediatric patients ≥ 8 years of age. CONTRAINDICATIONS - AED MODE: None known.
INDICATIONS FOR USE – MONITORING. AQUIRING 12-LEAD ECG: 12-lead electrocardiogram used to identify, diagnose, and treat patients with cardiac disorders and is useful in early detection and prompt treatment of patients with STEMI. MONITORING SPO2, SPCO, AND SPMET: Pulse oximetry indicated for use in any patient who is at risk of developing hypoxemia, carboxyhemoglobinemia, or methemoglobinemia. SpO2 monitoring may be used during no motion and motion conditions, and in patients who are well or poorly perfused. SpCO and SpMet accuracies have not been validated under motion or low perfusion conditions. MONITORING NONINVASIVE BLOOD PRESSURE: Intended for detection of hypertension or hypotension and monitoring blood pressure trends in patient conditions. NIBP monitoring not indicated for neonatal patients <1-month-old. MONITORING ETCO2: Used to detect trends in level of expired CO2, used for monitoring breathing efficacy and treatment effectiveness in acute cardiopulmonary care. MONITORING INVASIVE PRESSURE: Indicated for use in patients who require continuous monitoring of physiological pressures to rapidly assess changes in patient’s condition or response to therapy. May also be used to aid diagnosis. MONITORING CONTINUOUS TEMPERATURE: Indicated for use in patients who require continuous monitoring of body temperature. MONITORING CONTRAINDICATIONS: None known.
Operating Instructions provide important information to help you operate LIFEPAK 15. Become familiar with all terms and warnings. GENERAL DANGER: Explosion hazard. GENERAL/THERAPY/MANUAL DEFIBRILLATION WARNINGS and CAUTION: Shock or fire hazards. Possible patient skin burns and ineffective energy delivery. Possible device failure, damage, inability to deliver therapy, shutdown, loss of power during patient care, improper device performance. Possible electrical interference with device performance or with other equipment. Safety risk. Failure to detect change in ECG rhythm. Possible failure to detect out of range condition. Possible interference with implanted electrical device. Possible paddle damage. Possible incorrect energy delivery. CPR METRONOME WARNING: CPR delivered when not needed. SYNCHRONIZED CARDIOVERSION WARNING: Possible lethal arrhythmia. NONINVASIVE PACING WARNING: Possible inability to pace, interruption of therapy, ineffective pacing, and patient skin burns. PEDIATRIC ECG MONITORING AND MANUAL MODE THERAPY: Possible patient skin burns. AED WARNINGS: Possible misinterpretation of data or ECG misinterpretation. Pediatric patient safety risk. ECG MONITORING WARNING: Possible misinterpretation of ECG data. 12-LEAD ECG WARNINGS: Possible inability to obtain diagnostic quality 12-lead ECG or inaccurate diagnosis. Possible incorrect treatment with reperfusion therapy. SPO2, SPCO, AND SPMET WARNINGS AND CAUTION: Shock or burn hazard. Inaccurate pulse oximeter readings. Possible skin injury. Possible strangulation. Inaccurate SPO2, SPCO and/or SPMET readings. Possible equipment damage. NIBP MONITORING WARNINGS AND CAUTION: Possible loss of IV access and inaccurate infusion rate, circulation impairment or inaccurate blood pressure or oxygen saturation readings. Possible patient harm. Equipment damage. ETCO2 MONITORING WARNINGS AND CAUTION: Fire hazard. Possible inaccurate patient assessment or inaccurate CO2 readings. Possible strangulation. Infection hazard. Possible equipment damage. IP MONITORING WARNINGS: Possible inaccurate pressure readings, air embolism, blood loss or loss of sterility. Possible patient injury or equipment damage. Possible lethal arrhythmia. Increased intracranial pressure. TEMPERATURE MONITORING WARNINGS: Possible inaccurate temperature readings. Infection hazard. Possible strangulation. VITAL SIGN/ST SEGMENT TRENDS WARNING: Inaccurate interpretation of patient status.
U.S. Federal law restricts this device to sale by or on the order of a physician.
Please consult Operating Instructions at ifu.stryker.com or call 800.442.1142 for complete list of indications, contraindications, warnings, cautions, potential adverse events, safety and effectiveness data, instructions for use and other important information.
GDR 3338691_A
Brief summary of indications and important safety information
INDICATIONS FOR USE: LIFEPAK CR2 AED is indicated for use on patients 1 year of age or older in cardiopulmonary arrest. The patient must be unresponsive (unconscious), not breathing normally, and showing no signs of circulation (for example, no pulse, no coughing, or no movement). cprCOACH Feedback Technology in CR2 AED is indicated for use on cardiopulmonary arrest patients and provides CPR guidance in accordance with AHA Guidelines for patients 1 year of age or older. AED is intended for use by personnel who have been trained in its operation. Users should have received training in basic life support/AED, advanced life support, or a physician-authorized emergency medical response training program. The LIFEPAK CR2 Defibrillator is indicated to be used with the QUIK-STEP Pacing/ECG Defibrillation Electrodes and the LIFEPAK CR2 Lithium Battery.
CONTRAINDICATIONS: LIFEPAK CR2 AED is not indicated for patients who are conscious and responsive.
DANGER: Do not use LIFEPAK CR2 in presence of flammable gases or anesthetics. WARNINGS: LIFEPAK CR2 AED delivers up to 360 joules of electrical energy. Unless used properly by following AED’s visual and audio prompts, this electrical energy may cause serious injury or death. When instructed EVERYONE CLEAR, do not touch AED, patient, electrode pads or any material/fluid in contact with patient. Make sure no one is touching patient when AED shocks patient. Do not immerse AED in water or other fluids. Avoid spilling fluids on AED or its accessories. Do not store in presence of flammable gases, anesthetics or in direct contact with flammable material. Use care when operating close to oxygen sources. Turn off gas source or move it away from patient during defibrillation. Equipment operating in close proximity may emit strong electromagnetic interference (EMI) or radio frequency interference (RFI) which could affect performance of AED. Keep AED away from magnetic resonance imaging (MRI) equipment as it is unsafe. AED should not be used adjacent to or stacked with other equipment. Do not touch patient and USB connector on back of AED simultaneously. Replace battery immediately when AED indicates battery is low. Use only accessories specified by Physio-Control or Stryker. Using other manufacturers’ accessories may cause AED to perform improperly and may invalidate safety agency certification. Contact authorized service personnel for repair. QUIK-STEP electrode pads: Place pads so they adhere to skin completely. Do not allow pads to touch each other or any material on patient’s chest. Do not use damaged, expired, or dried-out pads. Dried out or damaged pads may cause electrical arcing and skin burns during defibrillation. Do not pull red handle to open electrodes until immediately before use. QUIK-STEP electrodes provided with CR2 are not compatible with LIFEPAK 500 device. Emergency medical personnel should not connect these electrodes to LIFEPAK 500 device.
CAUTIONS: Damaged batteries may leak and cause personal injury or equipment damage; handle with extreme care. Do not open device lid unnecessarily as this will reduce internal battery power.
POTENTIAL ADVERSE EFFECTS (for example, complications): Failure to identify shockable arrhythmia. Failure to deliver a defibrillation shock in presence of ventricular fibrillation (VF) or pulseless ventricular tachycardia, which may result in death or permanent injury. Inappropriate energy delivery which could cause failed defibrillation or post-shock dysfunction. Myocardial damage. Incorrectly shocking a pulse-sustaining rhythm and inducing VF or cardiac arrest. Bystander shock from patient contact during defibrillation shock. Interaction with pacemakers. Skin burns around electrode pad placement area. Allergic dermatitis due to sensitivity to materials used in electrode construction. Minor skin rash. Fire hazard in presence of high oxygen concentration or flammable anesthetic agents. EMI from AED impacting other devices especially during charge and energy transfers.
U.S. Federal law restricts this device to sale by or on the order of a physician.
Please consult Operating Instructions at ifu.stryker.com or call 800.442.1142 for complete list of indications, contraindications, warnings, cautions, potential adverse events, safety and effectiveness data, instructions for use and other important information.
GDR 3339803_A
Brief summary of indications and important safety information
INDICATIONS FOR USE: LIFEPAK CR Plus and LIFEPAK EXPRESS AEDs are indicated for use on patients in cardiac arrest. The patient must be unresponsive (unconscious), not breathing normally, and showing no signs of circulation (for example, no pulse, no coughing, or no movement). LIFEPAK AEDs are intended for use by personnel who have been trained in their operation. Users should have received training in basic life support/AED, advanced life support or a physician-authorized emergency medical response training program. The AEDs may be used with QUIK-PAK defibrillation pads only on adults and children who are 8 years old or more, or who weigh more than 55 lbs (25 kg). The AEDs may be used on children who are less than 8 years old or weigh less than 55 lbs (25 kg) with Physio-Control Infant/Child Reduced Energy Defibrillation Electrodes. The AEDs may be used with the CHARGE-PAK battery charger.
CONTRAINDICATIONS: Do not use LIFEPAK AEDs when the victim is conscious and responsive.
WARNINGS: AED: LIFEPAK AEDs deliver up to 360 joules of electrical energy. Unless properly used, this electrical energy may cause serious injury or death. Do not attempt to operate AED unless thoroughly familiar with the function of all controls, indicators, connectors, and accessories. When instructed “Do not touch patient,” “Stand by,” or “Everyone clear,” remain still, do not touch AED, patient, defibrillation pads or any material in contact with patient. Make sure no one is touching patient when AED shocks the patient. Performing CPR or otherwise handling or transporting the patient while AED is evaluating the heart rhythm can cause an incorrect or delayed diagnosis. Keep patient as still as possible. Do not immerse AED in water or other fluids. Avoid spilling any fluids on AED or its accessories. Do not use in presence of flammable gases or anesthetics. Use care when operating close to oxygen sources. Turn off gas source or move source away from patient during defibrillation. Contact authorized service personnel for repair. Equipment operating in close proximity may emit strong electromagnetic interference (EMI) or radio frequency interference (RFI) which could affect performance of AED. Keep AED away from magnetic resonance imaging (MRI) equipment as it is unsafe. Always keep a CHARGE-PAK battery charger in AED. Routinely check that AED is ready for use. Replace CHARGE-PAK battery charger and QUIK-PAK defibrillation pads after each use of AED. Insert only CHARGE-PAK battery charger into well of AED. Use only parts and accessories specified by Physio-Control or Stryker. Using other manufacturers’ accessories may cause AED to perform improperly and will invalidate safety agency certification. Using damaged or expired accessories may cause AED to perform improperly and may injure the patient or user. Defibrillation pads: Place defibrillation pads so they adhere to skin completely. Do not allow defibrillation pads to touch each other or any other material on patient’s chest. Do not use damaged, expired, or dried-out defibrillation pads. If you cannot determine a child’s age or weight, or if infant/child electrodes are not available, proceed with QUIK-PAK defibrillation pads.
CAUTIONS: If AED has been damaged, remove from use and contact qualified technician. Do not open device lid unnecessarily as this will reduce the internal battery power.
POTENTIAL ADVERSE EFFECTS (for example, complications): Failure to identify shockable arrhythmia. Failure to deliver a defibrillation shock in the presence of ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT), which may result in death or permanent injury. Inappropriate energy delivery which could cause failed defibrillation or post-shock dysfunction. Myocardial damage. Incorrectly shocking a pulse-sustaining rhythm and inducing VF or cardiac arrest. Bystander shock from patient contact during defibrillation shock. Interaction with pacemakers. Skin burns around the defibrillation pad placement area. Allergic dermatitis due to sensitivity to materials used in defibrillation pad construction. Minor skin rash. Fire hazard in the presence of high oxygen concentration or flammable anesthetic agents. EMI from the AED impacting other devices especially during charge and energy transfers.
U.S. Federal law restricts this device to sale by or on the order of a physician.
Please consult the Operating Instructions at ifu.stryker.com or call 800.442.1142 for the complete list of indications, contraindications, warnings, cautions, potential adverse events, safety and effectiveness data, instructions for use and other important information.
GDR 3336647_A
BRIEF SUMMARY OF INDICATIONS AND IMPORTANT SAFETY INFORMATION
INDICATIONS FOR USE: The HeartSine samaritan PAD 360P (SAM 360P) and HeartSine samaritan PAD 450P (SAM 450P) are indicated for use on victims of cardiac arrest who are exhibiting the following signs: unconscious, not breathing, without circulation (without a pulse). The AEDs are intended for use by personnel who have been trained in their operation. Users should have received training in basic life support/AED, advanced life support or a physician-authorized emergency medical response training program.
The AEDs are indicated for use on patients greater than 8 years old or over 55 lb (25 kg) when used with the adult Pad-Pak (Pad-Pak-01 or Pad-Pak-07). They are indicated for use on children between 1 and 8 years of age or up to 55 lb (25 kg) when used with the Pediatric-Pak (PAD-PAK-02).
CONTRAINDICATION: If the patient is responsive or conscious, do not use the HeartSine samaritan PAD to provide treatment.
WARNINGS: AEDs: • The HeartSine samaritan PAD (AED) delivers therapeutic electrical shocks that can cause serious harm to either users or bystanders. Take care to ensure that no one touches the patient when a shock is to be delivered. • Touching the patient during the analysis phase of treatment can cause interference with the diagnostic process. Avoid contact with the patient while the AED is analyzing the patient. The AED will instruct you when it is safe to touch the patient. • Do not delay treatment trying to find out the patient’s exact age and weight. If a Pediatric-Pak or an alternative suitable defibrillator is not available, you may use an adult Pad-Pak. • Disconnect non-defibrillation protected electronic devices or medical equipment from the patient before using the AED. • Do not use the AED in the vicinity of explosive gases, including flammable anesthetics or concentrated oxygen. • Do not use portable RF communications equipment closer than 12 in (30 cm) to any part of the AED. • Place pads at least 3.1 in (8 cm) away from a pacemaker. • The SAM 360P is a fully automatic defibrillator. When required, it will deliver a shock to the patient WITHOUT user intervention. • The SAM 450P CPR Rate Advisor is only intended to provide feedback on adult patients. If you treat a pediatric patient with the SAM 450P and an adult Pad-Pak, ignore any voice prompts regarding the rate of CPR. • Do not open or repair the AED under any circumstances as there could be danger of electric shock. If damage is suspected, immediately replace the AED. • Do not use any accessories other than those specified or provided by HeartSine Technologies as the AED may malfunction. Pad-Pak and Pediatric-Pak: • Do not use if the gel is dry. • Not for use on patients under 1 year old. • Pediatric-Pak is for use with children up to the age of 8 years or up to 55 lb (25 kg). DO NOT DELAY THERAPY IF YOU ARE NOT SURE OF EXACT AGE OR WEIGHT. • Pediatric-Pak is suitable for use only with HeartSine samaritan PADs with the adult/child symbol*. If the AED you are using does not have this label, use the adult Pad-Pak if no alternatives are available. • The use of the Pediatric-Pak will enable delivery of 50 J shocks to the pediatric patient. • It is advised that the Pediatric-Pak is stored separately when not in use. • Never charge, short circuit, puncture, deform, incinerate, heat above 85°C or expose contents of TSO (Aviation) Pad-Pak to water. Remove when discharged.
PRECAUTIONS: AEDs: • Check the AED periodically in accordance with the service and maintenance instructions provided in the user manual. • If you hear a warning message when you turn on your AED, consult Troubleshooting in the user manual. • Proper placement of the electrode pads is critical. Electrode pads must be at least 1 in (2.5 cm) apart and should never touch one another. • Do not use electrode pads if pouch is not sealed. • Do not pull green tab on Pad-Pak during set-up.• If a non-shockable rhythm is detected, the AED will end its ready to shock condition if it previously decided to shock. • Use of the AED outside the operating and storage ranges specified in the user manual may cause the AED to malfunction or reduce the shelf life of the Pad-Pak. • Do not immerse any part of the AED in water or any type of fluid. • Do not clean the AED with abrasive materials, cleaners or solvents. • Do not turn on the AED unnecessarily as this may reduce the standby life. • Do not use any unauthorized accessories with the AED as it may malfunction if non-approved accessories are used. • Dispose of the AED in accordance with national or local regulations. • Check with the relevant local government health department for information about any requirements associated with ownership and use of a defibrillator in the region where it is to be used. Pad-Pak and Pediatric-Pak: • Single use only. Reuse may cause AED to be unable to deliver therapy leading to a failure to resuscitate or lead to cross-infection between patients. • Do not use if open or damaged. • Check expiration date. • It is advised that a spare Pad-Pak be stored with AED in rear section of carry case. Saver EVO Software: • Download the complete HeartSine samaritan PAD memory prior to erasing it. This information should be stored safely for future reference. Ensure that only the events you want to delete have been selected prior to deleting. Once deleted from your computer’s memory, events cannot be regenerated, and all information will be lost.
POTENTIAL ADVERSE EFFECTS: The potential adverse effects (e.g., complications) associated with the use of an automated external defibrillator include, but are not limited to, the following: o Failure to identify shockable arrhythmia o Failure to deliver a defibrillation shock in the presence of VF or pulseless VT, which may result in death or permanent injury o Inappropriate energy which could cause failed defibrillation or post-shock dysfunction o Myocardial damage o Fire hazard in the presence of high oxygen concentration or flammable anesthetic agents o Incorrectly shocking a pulse-sustaining rhythm and inducing VF or cardiac arrest o Bystander shock from patient contact during defibrillation shock o Interaction with pacemakers o Skin burns around the electrode placement area o Allergic dermatitis due to sensitivity to materials used in electrode construction o Minor skin rash
CAUTION: U.S. Federal law restricts the SAM 360P and SAM 450P AEDs to sale by or on the order of a physician.
Please consult the user manuals at heartsine.com for the complete list of indications, contraindications, warnings, precautions, potential adverse events, safety and effectiveness data, instructions for use and other important information.
H009-025-005-AC